



Media
[News] Hua Medicine Announces Potential Effects of the Combination of Dorzagliatin and SGLT-2 Inhibitor at 2024 Annual Scientific Sessions



June 24, 2024 - China, Shanghai
Hua Medicine (the "Company", HKEx stock code: 2552) announced today that the Company and its wholly-owned subsidiary, Nanjing AscendRare Pharmaceutical Technology Co., Ltd.(AscendRare), presented a number of basic research findings at the 84th American Diabetes Association (ADA) Annual Scientific Sessions. A basic research showed that dorzagliatin reduces glucose level by enhancing the secretion of insulin and GLP-1, and that the combination of dorzagliatin and the SGLT-2 inhibitor canagliflozin has superior glucose-reducing effects than monotherapy, providing a synergistic effect. Additional basic research suggests that dorzagliatin has potential in diabetes prevention.
Dorzagliatin is the world's first glucokinase activator independently developed by Hua Medicine, aiming to stabilize the imbalanced blood glucose levels in patients with type 2 diabetes by repairing the impaired function and expression of glucokinase and enhancing glucose sensitivity in patients with type 2 diabetes. Previous basic and clinical research has shown that its mechanism of action also includes improving GLP-1 secretion, restoring pancreatic islet function and enhancing insulin sensitivity in the liver. Canagliflozin is an SGLT-2 inhibitor. This basic research aims to assess the regulation of glucose homeostasis in mice by the combination of the two drugs, laying the foundation for clinical combination therapy.
The research began with the separate administration of the two drugs to mice to explore the mechanism of action of the two drugs. The research showed that canagliflozin improves glucose control by reducing the renal threshold for glucose reabsorption without affecting hormone secretion, and dorzagliatin exhibited comparable glucose-lowering effects by elevating insulin and GLP-1 secretion. In the research for combined medications, the mechanisms of action of both drugs was preserved, and the effects of the combination observed at all time points (random, overnight fasting and 3 hours refeeding) were superior to those of dorzagliatin or canagliflozin alone, providing a synergistic effect. Previous clinical studies by Hua Medicine have also shown that dorzagliatin in combination with the SGLT-2 inhibitor Empagliflozin was also more effective than monotherapy. In addition, the combination was found to stimulate glucagon secretion, which is expected to bring cardiovascular benefits.
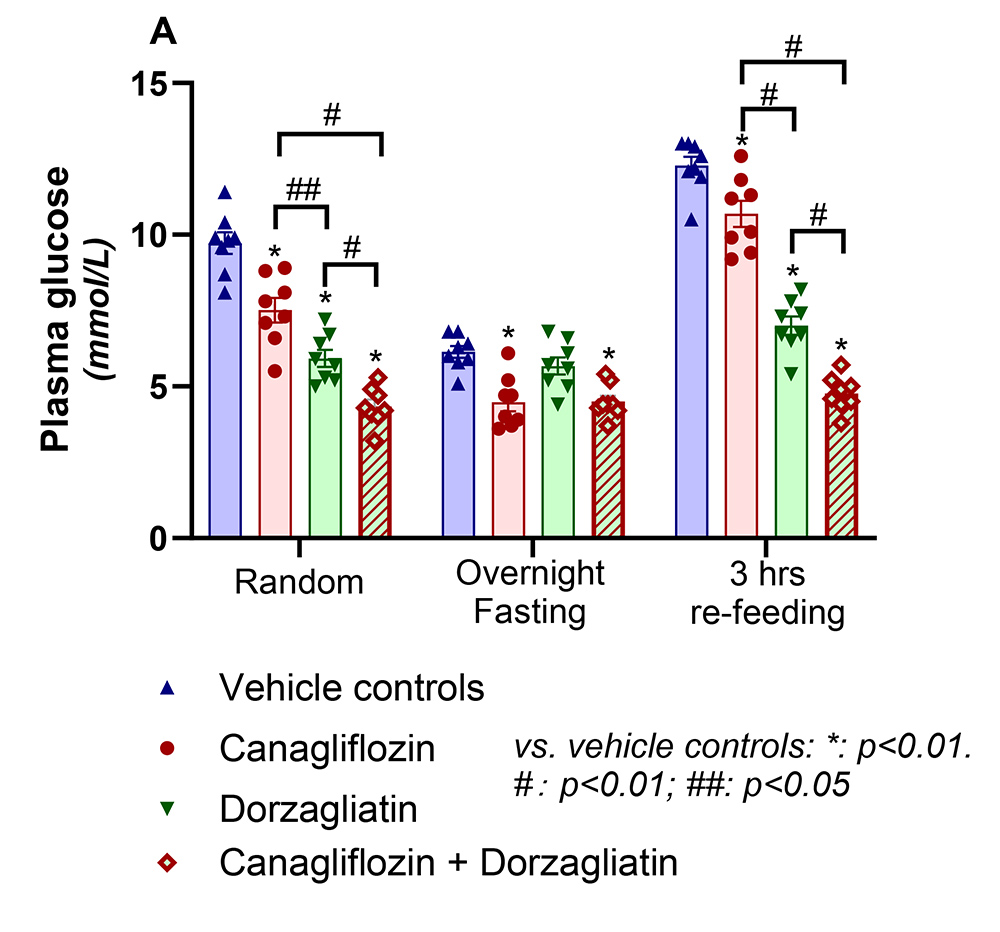
The effects of 5-days administration of canagliflozin, dorzagliatin or combination of two on glucose levels (random, overnight fasted and 3-hrs re-feeding)
This research provides a basis for basic research to guide doctors in clinical use of the drug, and also explores new possibilities for combination therapy in terms of renal function protection and cardiovascular benefits.
The Company also reported on basic research jointly conducted with the Chinese University of Hong Kong to explore the opportunity to reverse patients with impaired glucose tolerance (IGT) to normal glucose tolerant (NGT) in China. By studying the effects of single dose (50 mg) dorzagliatin on insulin secretion and glucagon suppression triggered by glucose during hyperglycemic clamp in patients with IGT and NGT, the research found that dorzagliatin was able to increase second-phase insulin secretion of patients with IGT and basal insulin secretion of NGT, while improving the glucose-suppressed glucagon secretion in NGT. The research suggests that dorzagliatin has the mechanistic potential to reverse IGT to NGT, thereby achieving the aim of preventing diabetes.
In addition, Hua Medicine and AscendRare presented other basic research results through poster presentations, including the research on canagliflozin’s stimulation of glucagon secretion through direct action on α-cells under specific conditions. Glycine enhances glucose-stimulated insulin secretion through the involvement of the NMDA receptor pathway, providing a new direction for the research of islet-central nervous system. The above studies will further explore the potential clinical applications of dorzagliatin and expand Hua Medicine's future product pipeline and disease areas.
About Hua Medicine
Hua Medicine is an innovative drug development and commercialization company based in Shanghai, China, with companies in the United States and Hong Kong, China. Hua Medicine is focused on developing novel therapies for patients around the world with unmet medical needs. Based on global resources, Hua Medicine teams up with high-caliber people to develop breakthrough technologies and products, which contribute to global innovation in diabetes care. Hua Medicine's cornerstone product HuaTangNing (华堂宁®) (dorzagliatin tablets) targets the glucose sensor glucokinase, restores glucose sensitivity in Type 2 diabetes (T2D) patients and stabilizes imbalances in blood glucose levels in patients. HuaTangNing was approved by the National Medical Products Administration (NMPA) of China on September 30, 2022. It can be used alone or in combination with metformin in metformin hydrochloride-tolerated T2D patients. For patients with chronic kidney disease (CKD), no dose adjustment is required. It is an oral hypoglycemic drug that can be used by patients with T2D with varying degrees of renal function impairment (including end-stage renal impairment without dialysis). Hua Medicine has partnered with Bayer, a leading global pharmaceutical company, to commercialize HuaTangNing (华堂宁®) in China, benefiting diabetes patients and their families.
About AscendRare
Nanjing AscendRare Pharmaceutical Technology Co., Ltd is a wholly owned subsidiary of Hua Medicine, situated within the Nanjing Biotech and Pharmaceutical Valley in the Jiangbei New District. The company's primary focus lies in conducting research and development (R&D) activities pertaining to pharmaceuticals, specifically targeting the treatment of rare metabolic diseases. The overarching goal is to develop and deliver efficacious therapeutic interventions for rare metabolic diseases, with a particular emphasis on newborn hypoglycemia. Additionally, the company offers supplementary diagnostic services for rare diseases, encompassing functional investigations of mutant genes. The company has implemented an extensive pre-clinical research and development (R&D) technology platform. This platform encompasses various components such as computer-aided drug screening, compound optimization and synthesis, target protein function testing, compound screening, drug screening based on islet function, and drug efficacy determination using disease animal models. The company has also established R&D pipelines targeting different objectives. The company has been designated as a "Private Technology Enterprise," a "Small and Medium-sized Technology Enterprise," and an "Innovative Small and Medium-sized Enterprise." It has collaborated with numerous domestic and international universities and scientific research institutes to consistently broaden the investigation of illness mechanisms alongside the development of groundbreaking pharmaceuticals.
For more information
Hua Medicine
Website: www.czfubang.com
Investors
E-mail: ir@czfubang.com
Media
E-mail: pr@czfubang.com



沪ICP备99665955号-1
 沪公网安备 31011502013809号 Privacy Statement Terms of Use
沪公网安备 31011502013809号 Privacy Statement Terms of Use













